UNAIR NEWS – Coronavirus plague or Covid-19 has been a serious problem in Indonesia. The number of cases continues to grow and it requires the administration’s swift response in making policies related to Covid-19 handling.
After issuing policies related to physical distancing and working at home, this time the government made a new decision regarding the drugs used. The choice of the Indonesian government fell on Avigan and Chloroquine. Although still quite foreign to the ears of the public, both drugs have been proven to have a mechanism that is needed to deal with Covid-19.
One of the lecturers from Faculty of Pharmacy, Universitas Airlangga (UNAIR), Mahardian Rahmadi, S.Si., M.Sc., Ph.D., Apt. conveyed some information related to the two drugs.
“Avigan is a trade name for the favipiravir drug developed by Toyama Chemical, a group from Fujifilm. Favipiravir is used to treat RNA virus infections,” Mahardian explained.
Favipravir has been approved by the Japanese Food and Drug Administration (BPOM) since 2014 to treat various viruses that are not responsive to existing antivirals. Furthermore, Favipravir has also been approved by Food and Drug Administration (FDA) United States to be used as an antiviral to treat influenza.
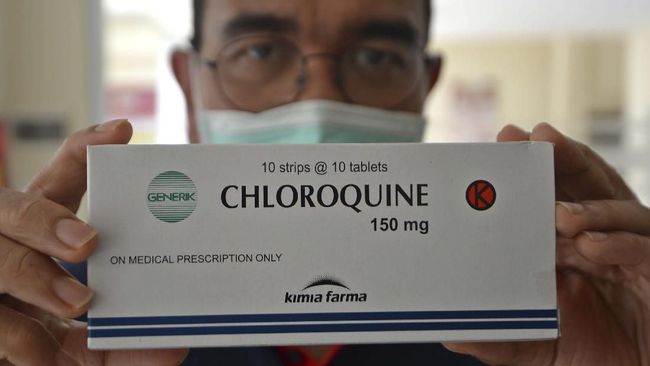
“Meanwhile, Chloroquine is a drug that has long been used to treat parasite infections, especially plasmodium which causes malaria,” explained Mahardian.
“In previous invitro studies (not in human or animal living creatures), chloroquine is effective as an antiviral against various types of RNA viruses including SARS-CoV1, hepatitis A virus, hepatitis C virus, influenza virus A and B, bird flu virus (H5N1), Dengue Virus, Zika Virus and others, “he added.
The two drugs were chosen to treat Covid-19 because according to several studies, Mahardian revealed that clinical trial results in various countries, favipiravir and chloroquine were effective enough to overcome SARS-Cov2 virus infection (the virus that causes covid-19), although to ensure it more tests and more patients are needed. Chloroquine is also suspected to have anti-inflammatory and immunomodulatory activities that can help the recovery process in Covid-19 patients.
As a pharmacist and academic, Mahardian claimed to agree with the choices made by the government. According to him, with promising pre-clinical trial data and evidence that both drugs have been widely used by various countries, Avigan and Chloroquin are quite good choices.

“Safety is also assured because both drugs have undergone various stages of clinical trials and have long been used for other diseases,” he added.
Chloroquine panic buying phenomenon
After the announcement of Avigan and Chloroquine as drugs to deal with Covid-19, a new phenomenon emerged in the community. There was a panic buying for chloroquine as supplies at home. Mahardian also expressed his opinion regarding the phenomenon.
“The two drugs should only be used in hospitals, under the supervision of doctors and other health professionals. It’s too risky for the public to use these drugs without doctor’s prescription,” said the lecturer in Department of Clinical Pharmacy UNAIR.
Mahardian also said that the two drugs should only be given to patients who are positive for Covid-19 with close supervision from medical workers.
Just like drugs in general, Chloroquine can also cause side effects. For this reason, the public must be very careful and not take drugs carelessly.(*)
Author: Sukma Cindra Pratiwi
Editor: Binti Q Masruroh